 As of April 2025, The Indian pharmaceutical industry is the world’s 3rd largest by volume and 11th largest in terms of value.
As of April 2025, The Indian pharmaceutical industry is the world’s 3rd largest by volume and 11th largest in terms of value.
- India is globally the largest supplier of generic drugs, accounting for 20% of global supply.
- India is also one of the biggest suppliers of low-cost vaccines in the world.
Medical Devices and Pharmaceutical Industry:
i.India is the fourth largest medical device market in Asia, after Japan, China and South Korea.
ii.India is among the top 20 global medical device markets in the world.
iii.The total annual turnover of Pharmaceuticals in Financial Year (FY) 2023-24 was Rs.4,17,345 crores, out of which Rs.2,19,439 was from exports.
Foreign Direct Investment (FDI):
i.As per FDI Policy 2020, up to 100% foreign investment is allowed under the automatic route in meditech activities.
ii.In Pharmaceuticals, up to 100% FDI in greenfield projects and up to 74% FDI in brownfield projects is allowed under the automatic route.
iii.In the FY of 2024-25, from April 2024 to December 2024, FDI inflows (in both pharmaceuticals and medical devices) has been ₹11,888 Crores. Also, the Department of Pharmaceuticals has approved 13 FDI proposals worth ₹7,246.40 Crore for brownfield projects during FY 2024-25.
Production Linked Incentive Scheme (PLI):
i.The PLI Scheme, launched in 2020 by the Government of India, is a transformative initiative aimed at boosting domestic manufacturing, attracting investments, reducing reliance on imports and increasing exports.
ii.The Department of Pharmaceuticals administers three PLI schemes to enhance manufacturing capabilities. These include:
- PLI Scheme for Pharmaceuticals:
i.This was approved by the Union Cabinet on 24 February 2021 with a financial outlay of ₹15,000 crores, offers financial incentives over six years (FY 2022–23 to FY 2027–28) to 55 selected manufacturers.
ii.The scheme supports the production of high-value pharmaceutical products across 3 categories:
| Category 1 | Category 2 | Category 3 |
|---|---|---|
| Biopharmaceuticals, complex generic drugs, patented drugs or those nearing patent expiry, gene therapy drugs, orphan drugs, and complex excipients. | Active Pharmaceutical Ingredients (APIs), Key Starting Materials (KSMs), and Drug Intermediates (DIs). | Repurposed drugs, autoimmune drugs, anti-cancer drugs, anti-diabetic drugs, cardiovascular drugs, and in-vitro diagnostic (IVD) devices. |
- PLI Scheme for KSMs, DIs, and APIs:
i.It was launched on 20 March 2020, with a financial outlay of ₹6,940 crore for the period FY 2020-21 to FY 2029-30.
ii.The objective of the scheme is to promote domestic manufacturing of 41 identified bulk drugs to address their high import dependence.
Achievements under the scheme
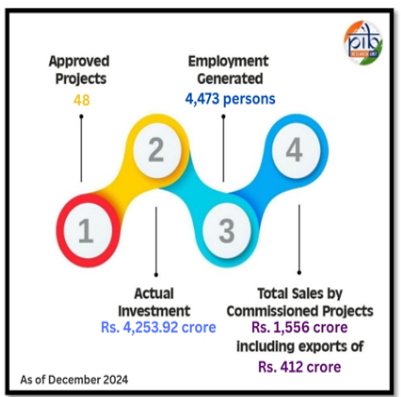
- PLI Scheme for Medical Devices:
i.The scheme provides financial incentives to manufacturers in key segments such as radiology, imaging, cancer care, and implants.
ii.The period of the scheme is from financial year 2020-21 to financial year 2027-28 with total financial outlay of Rs. 3,420 crore. The following is the incentive period and rate:
| Category of Applicant | Inventive Period | Incentive Rate |
|---|---|---|
| Category A | FY 2022-23 to FY 2026-27 | 5% limited to Rs.121 crore per applicant |
| Category A | FY 2022-23 to FY 2026-27 | 5% limited to Rs.40 crore per applicant |
Other Important Schemes:
i.The Promotion of Bulk Drug Parks Scheme (FY 2020–21 to FY 2025–26) aims to set up parks with top-class shared infrastructure to lower manufacturing costs and boost self-reliance in bulk drug production.
- Financial assistance is capped at ₹1,000 crore per park or 70% of the project cost (90% for Northeastern and Hilly States), with a total outlay of ₹3,000 crore.
ii.The Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP) aims to provide affordable, quality generic medicines to all, ensuring easy access to essential drugs across India.
- As of April 8, 2025, there are a total of 15,479 Jan Aushadi Kendras across the country.
iii.The Strengthening of Pharmaceuticals Industry Scheme (SPI) scheme is a Central Sector Scheme (CSS) with an outlay of Rs.500 Crores with the scheme period from FY 2021-22 to FY 2025-26. The objective of the scheme is:
- To strengthen the existing infrastructure facilities in order to make India a global leader in Pharma Sector.
- To upgrade the production facilities of Small and Medium Enterprise (SMEs) and Micro, Small and Medium Enterprises (MSMEs), to meet national and international regulatory standards.
- To promote knowledge and awareness in and about the Pharmaceutical and Medical Devices Industry.
iv.Ministry of Chemicals and Fertilizers in 2019 launched mobile application “Janaushadhi Sugam” and “Jan Aushadhi Suvidha Oxo-Biodegradable Sanitary Napkin” available at only One Rupee per pad
Note:
India has been the United Nations International Children’s Emergency Fund (UNICEF’s) largest vaccine supplier for the past six to seven years, contributing 55% to 60% of total volume procured contributing 99%, 52% and 45% of the World Health Organisation (WHO) demand for Diphtheria, Pertussis and Tetanus (DPT), Bacillus Calmette–Guérin (BCG) and the measles vaccines, respectively




